EXPERTSMINDS.COM ACCEPTS INSTANT AND SHORT DEADLINES ORDER FOR ACTIN ASSIGNMENT - ORDER TODAY FOR EXCELLENCE!
Actin Assignment
Question:
Select a protein from the list (Protein and Reference/Brief Description) provided to study using the Deep View program and the primary literature on the protein.
Solution:
Introduction
Actin is considered to be highly conserved protein that has about six mammalian isoforms that are encoded by six different genes, namely, α-cardiac, α-smooth, α-skeletal, γ-smooth, along with 2 more ubiquitously expressed cytoplasmic or non-muscle isoforms that are known as γ-cytoplasmic and β-cytoplasmic (Somlyo&Siegman, 2012). Uncomplexed actin (PDB = 1j6z) is obtained from the organism Oryctolagus cuniculus and is a globular protein which is associated with the performing crucial cellular functions. Actin is considered the main component of the thin filament present in the muscle cells aned of the cytoskeletal system that is present in the non-muscle cells. It takes part in varied kinds of biological functions (Otterbein, Graceffa, & Dominguez, 2001). The dynamics as well as the polarity of the actin filaments are controlled by the conformational change that are coupled with the hydrolysis of the adenosine 59-triphosphate (ATP) by the kind of mechanism that are yet to be elucidated. In the following discussions, the various aspects of Actin protein have been elucidated.
SAVE YOUR HIGHER GRADE WITH ACQUIRING ACTIN ASSIGNMENT HELP & QUALITY HOMEWORK WRITING SERVICES OF EXPERTSMINDS.COM
Background Information of the Protein
What is the function of the protein?
Function of the Protein
Actin 1j6z performs the function of maintaining the shape of the cells, supporting the process of cytokinesis during the process of cellular division, supporting the processes of cellular signalling, networking with the myosin for performing muscular contractions, and performing the function of a transport track for other proteins (Antonious, n.d.). Apart from these, it is also performs several of the biochemical functions and these include bindings of myosin heavy chain, troponin I, protein, calcium ion, actin monomer, calcium dependent protein, titin, protein domain specific, nucleotide, magnesium ion, identical protein, ATP, and tropomysin binding (Protein Data Bank in Europe, 2012). The biological process that is associated with Actin 1j6z is the positive regulation of the gene expressions (Protein Data Bank in Europe, 2012).
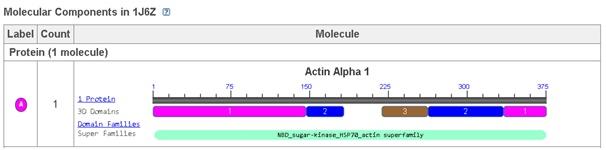
Figure 1: Molecular component of Actin 1j6z
Why is it of Interest
Uncomplexed Actin 1j6z is fundamentally a crystallised protein that is present in a monomeric form. The protein is comprised of four major subdomains and these consists of alpha helices, beta turns and beta sheets. The subdomains 1 and 3 comprises of 5 stranded bet pleated sheets. On the other hand, the subdomains 2 and 4 comprises of the anti-parallel beta sheets (Antonious, n.d.). The subdomains of the protein further meet at the centre of the protein and at an adenosine diphosphate ligand. Surrounding this ligand is a binding pocket that in turn comprises of the residues, namely, Lys-336, Tyr-306, Met-305, Thr-303, Glu-214 and Lys-213. The uncomplexed actin 1j6z present in the ADP state comprises of various alpha sheets, random coils, turns and beta sheets. In addition, certain of the amino acids present in them exhibit hydrogen bonding as well (Antonious, n.d). Due to the dynamic property and function of uncomplexed actin 1j6z, this protein is of interest and therefore has been considered for the current discussions.
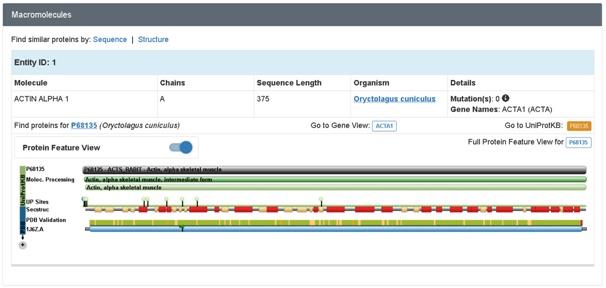
Figure 2: Macromolecule of Actin 1j6z
Where is It Synthesized or Active
The actin comprises of hsp70 molecular chaperones, sugar kinases and hexokinase and the same appears to undergo a conformational change which is coupled to the hydrolysis of ATP. Within the actin, the conformational change has the potential of playing a crucial role in the filament dynamics. The ADP-actin filaments exhibit a higher susceptibility for the proteins of depolymerisation properties. With the inorganic phosphate (Pi) or the Pianalog, the ADP-actin filaments stabilise and the same is presumed to take place due to the mimicking of the ADP-Pistate. It has been determined that the different properties of ADP-actin as well as ADP-Pi-actin filaments have correlations with the differences in their tertiary structures (Otterbein, Graceffa, & Dominguez, 2001).
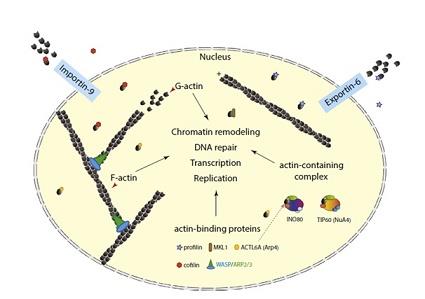
Figure 3:Actin in the nuclear compartment.
The crystal structures of ATP and the ADP bound actin complexed with the DNase I are highly similar. However, the ADP structure was acquired after the hydrolysis of the ATP inside the crystals which were initially grown on the ATP state. Therefore, it is possible that the structure was controlled within the ATP state by the interactions or crystal contacts between the actin subdomain 2 and the DNase I (Otterbein, Graceffa, & Dominguez, 2001).
ORDER NEW ACTIN ASSIGNMENT & GET 100% ORIGINAL SOLUTION AND QUALITY WRITTEN CONTENTS IN WELL FORMATS AND PROPER REFERENCING.
Structural Information of the Protein
How many subunits are present?
The details regarding the structural information of the uncomplexed actin 1j6z have been detailed as presented below. The following image is the front view of the 1j6z uncomplexed actin.
Figure 4:PDB 1j6z coloured by the chain and viewed from the front
Subunits
The subdomains of the uncomplexed actin 1j6z encompass Nucleotidyltransferase in the domain 5, Actin in the Chain A of domain 4 and Actin in the Chain A of domain 2.
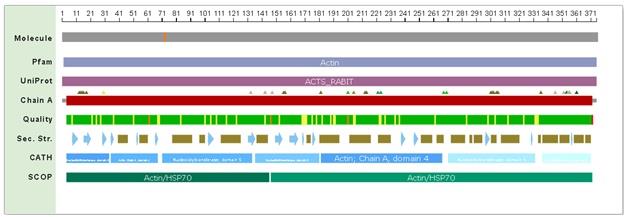
Figure 5: Compact PDB Sequencer View
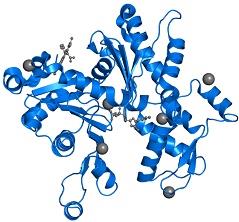
Figure 6: Front view of the uncomplexed actin 1j6z
The PDB16jz comprises of 1 copy of the Actin, along with the alpha skeletal muscle in the assembly 1. These proteins have been clearly highlighted in the image that has been presented in figure 6. The subdomains 1 and 3 comprises of 5 stranded bet pleated sheets. On the other hand, the subdomains 2 and 4 comprises of the anti-parallel beta sheets (Antonious, n.d.).The subdomains of the protein further meet at the centre of the protein and at an adenosine diphosphate ligand. Surrounding this ligand is a binding pocket that in turn comprises of the residues, namely, Lys-336, Tyr-306, Met-305, Thr-303, Glu-214 and Lys-213.
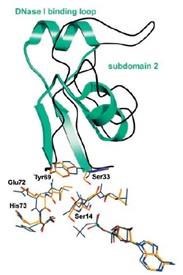
Figure 7: Actin Subdomain 2
What are the secondary structural elements of the protein?
Secondary Structural Elements of the Protein
Actin 1j6z performs the function of maintaining the shape of the cells, supporting the process of cytokinesis during the process of cellular division, supporting the processes of cellular signalling, networking with the myosin for performing muscular contractions, and performing the function of a transport track for other proteins (Antonious, n.d.).
a. How many domains are present?
Domains Present
Uncomplexed Actin 1j6z is fundamentally a crystallised protein that is present in a monomeric form. The protein is comprised of four major subdomains and these consists of alpha helices, beta turns and beta sheets.The subdomains 1 and 3 comprises of 5 stranded bet pleated sheets. On the other hand, the subdomains 2 and 4 comprises of the anti-parallel beta sheets (Antonious, n.d.).The dynamics as well as the polarity of the actin filaments are controlled by the conformational change that are coupled with the hydrolysis of the adenosine 59-triphosphate (ATP) by the kind of mechanism that are yet to be elucidated. In the following discussions, the various aspects of Actin protein have been elucidated.
DO YOU WANT TO EXCEL IN ACTIN ASSIGNMENT? HIRE TRUSTED TUTORS FROM EXPERTSMINDS AND ACHIEVE SUCCESS!
a. Which domains / folds / motifs are present?
Domain or Folds or Motifs Present
Actin has the ability of spontaneously acquiring a large part of the tertiary structure and although the way it acquires its completely functional form from the newly produced native structure, which is special as well as unique with respect to the protein chemistry. The manner in this special route is formed is required is for avoiding the presence of the incorrect fold of the actin monomer that could be toxic and they have the ability of acting as ineffective polymerization terminators. The four subdomains of the uncomplex actin 1j6z has the ability of folding the structure in the manner that is required by the protein. The DNase I binding loop is folded to form the alpha helix in the ribbon representation structure and the same is located towards the upper position of the subdomain 2. The ADP is bound and positioned at the centre of the molecule and at the same position, the four actin subdomains meet as well. It has been suggested from the conformational differences that have been observed between the ADP structure as well as the ATP actin structures that the nucleotide differences present in these locations have the ability of providing a mechanism that facilitates the change of the orientations of the actin subdomains that are related to each other. Four of the Ca2+ ions bound themselves to the actin monomer in the crystals and one of the calcium ions, which is also termed as the catalytic or primary ion, is bound in close association with the nucleotide. The rest of the three calcium ions bind themselves with the subdomains 1, 2 and 4 that are present on the surface of the molecule and thereby may correspond with the secondary cation-binding sites present in the actin 1j6z.
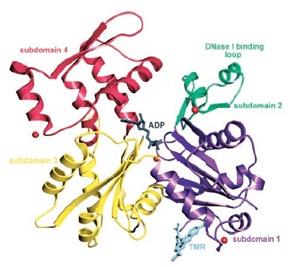
Figure 8: Ribbon representation of the structure of the uncomplexed actin 1j6z
How do these individual domains impart a complex function to the protein as a whole?
Individual Domains Impart a Complex Function to The Protein as a Whole
Figure 9: Actin subunit from the filament model of Oda in the atomistic representations
The four subdomains of the uncomplexed actin 1j6z have the capacity of binding with the various ions that they are compatible with to perform the functions that are respective to those ions and thereby facilitate the entire process that are associated with the fundamentals of the uncomplexed actin 1j6z protein.
Specific descriptions of molecular interactions of the protein.
Molecular Interactions of the Protein
Describe three to four molecular interactions that are important for the protein's structure function.
Actin-Actin Interaction
Actin is considered to be a very plentiful and conserved eukaryotic protein and the eukaryotic cells have advanced themselves for the internal supporting structures that are also known as the cytoskeletons. Fundamentally, within the cytoskeleton structures, there exists the microtubules, actin filaments, and the intermediate filaments (Ünlü, 2014). The 3D globular structure of the monomeric actin functions is regulated by the protein-protein interactions. Actins has the most critical function of interacting with itself so as to form the F-actin filaments. As the actin conducts its cellular functions with the help of its filamentous form, therefore, it is important to acquire knowledge regarding the filaments of the actins so as to gain an understanding of the mechanistic function of the actin (Ünlü, 2014).
Figure 10: Starting structure of the Actin S1 complex along with the five actin monomers as well as the S1.
The ADP-actin filaments exhibit a higher susceptibility for the proteins of depolymerisation properties. With the inorganic phosphate (Pi) or the Pianalog, the ADP-actin filaments stabilise and the same is presumed to take place due to the mimicking of the ADP-Pi state.The actin comprises of hsp70 molecular chaperones, sugar kinases and hexokinase and the same appears to undergo a conformational change which is coupled to the hydrolysis of ATP (Otterbein, Graceffa, & Dominguez, 2001).
Figure 11: The interaction of Actin - Actin at ADP1380v
GET GUARANTEED SATISFACTION OR MONEY BACK UNDER ACTIN ASSIGNMENT HELP SERVICES OF EXPERTSMINDS.COM - ORDER TODAY NEW COPY OF THIS ASSIGNMENT!
Fenvalarate - Actin Interaction
Fenvalarate is a synthetic pyrethroid which is utilised in the area of various domestic as well as agricultural purposes. The application of the same is associated with the insecticidal activity as well as phytotoxicity and the low mammalian toxicity. It has the ability of easily being biodegradable as opposed to the organic chlorides and the organic phosphates (Karunakar, Krishnamurthy, Girija, Krishna, Vasundhara, Begum, & Syed, 2011). Actin, which is the most abundantly found intercellular cytoskeletal protein amongst the eukaryotic cells are found to have interactive behaviour with the Fenvalarate. Within the neuronal cells, the actin cytoskeleton is associated with the significant cellular activities like the intracellular transportation, cellular division, neuronal signalling, and several other similar functioning (Karunakar, Krishnamurthy, Girija, Krishna, Vasundhara, Begum, & Syed, 2011). It has been determined that the ADT package of the hydrogen atoms when added cause the non-polar hydrogens to merge with the lone pairs and each of the atom within the macromolecule is assigned with a Gasteiger partial charge (Karunakar, Krishnamurthy, Girija, Krishna, Vasundhara, Begum, & Syed, 2011).
The interaction of the Fenvalarate with that of the active sites of the actin 1j6z has been identified to present a binding affinity of anout -7.71kcal/mol and the same when is compares with that of the ATP binding energy, then that is acquired to be -6.25kcal/mol (Karunakar, Krishnamurthy, Girija, Krishna, Vasundhara, Begum, & Syed, 2011). This illustrates that the Fenvalarate has a strong affinity as well as toxic influence against the actin 1j6z as opposed to the ATP binding. The structural changes that are associated with the active site of Fenvalarate with that of the actin has been determined to be responsible for the string binding affinity as opposed to the ATP binding energy (Karunakar, Krishnamurthy, Girija, Krishna, Vasundhara, Begum, & Syed, 2011). And this in turn signifies that the Fenvalarate is moderately toxic to the mammals that have the ATP binding pockets and the same is not very different from the ATP binding processes. The observation, therefore, reinforces that the Fenvalarate indeed is toxic to the smaller insects comprising of the uncomplex 1j6z, while the same is not very toxic to the human uncomplex 1j6z.
Figure 12: The interaction of Fenvalarate - Actin at h SER338 v
It has been observed that the presence of the oxygen of the alcohol moiety of the fenvalarate hydrogen bonding along with the nitrogen of the Gly302 of actin has a bond distance of 2.187 along with a hydrogen bond formation in the zeta position of the Lys336 that has a bond distance of 1.939.
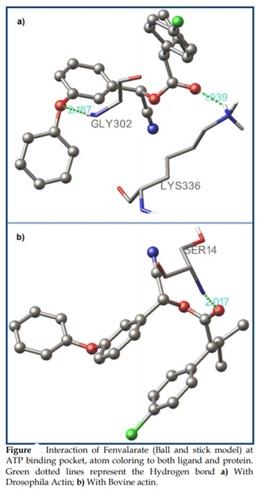
Figure 13: Interaction of Fenvalarate and actin 1j6z
Figure 14: The entire atomic structure of the uncomplexed action 1j6z in undifferentiated form Retrieve the primary amino acid sequence of your protein.
Amino Acid Sequence of the Protein
Full primary amino acid sequence
10 20 30 40 50
MCDEDETTALVCDNGSGLVKAGFAGDDAPRAVFPSIVGRPRHQGVMVGMG
60 70 80 90 100
QKDSYVGDEAQSKRGILTLKYPIEHGIITNWDDMEKIWHHTFYNELRVAP
110 120 130 140 150
EEHPTLLTEAPLNPKANREKMTQIMFETFNVPAMYVAIQAVLSLYASGRT
160 170 180 190 200
TGIVLDSGDGVTHNVPIYEGYALPHAIMRLDLAGRDLTDYLMKILTERGY
210 220 230 240 250
SFVTTAEREIVRDIKEKLCYVALDFENEMATAASSSSLEKSYELPDGQVI
260 270 280 290 300
TIGNERFRCPETLFQPSFIGMESAGIHETTYNSIMKCDIDIRKDLYANNV
310 320 330 340 350
MSGGTTMYPGIADRMQKEITALAPSTMKIKIIAPPERKYSVWIGGSILAS
360 370
LSTFQQMWITKQEYDEAGPSIVHRKCF
Figure 15: Primary sequence with a length of 377 residues - derived from Uniprot.
Secondary structures positions

Figure: Secondary structural elements position of Actin.
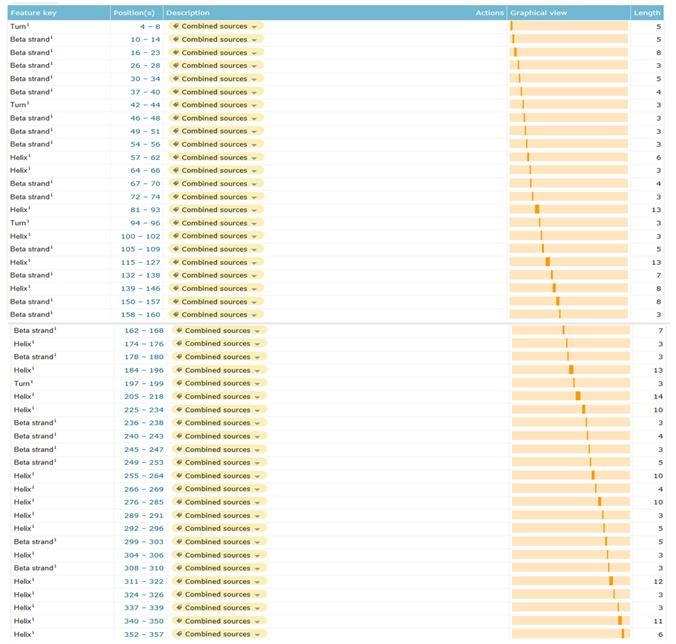
Figure 16:Secondary structural elements position of Actin

Figure 17:Secondary structural elements in the Actin
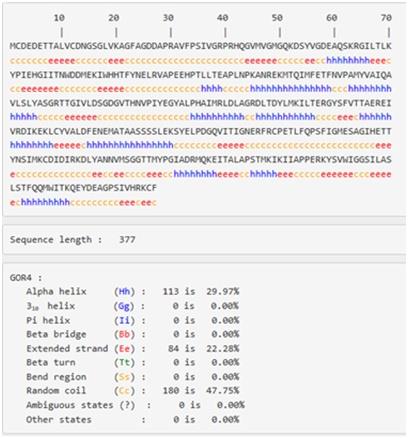
Figure 18:Positions of secondary structure elements within the sequence of actin
42% of the secondary structure of actin is alpha helices (22 helices; 159 residues) , whereas the beta sheets are taking 3% (19 strands; 70 residues) of its structure, and there is also a four turns various in length found in structure as in the figure 16.
DO WANT TO HIRE TUTOR FOR ORIGINAL ACTIN ASSIGNMENT SOLUTION? AVAIL QUALITY ACTIN ASSIGNMENT WRITING SERVICE AT BEST RATES!
Classification of Actin (membrane, mixed, fibrous, globular)
PRED-CLASS: Classi?cation of protein sequences
Sequence:
Classified in the globular protein class.
Figure 19:Classi?cation of actin as a globular protein.
Trans-membrane regions
Actin is classified as a globular protein and hence, it has no transmembrane regions, and this is confirmed in following figure.
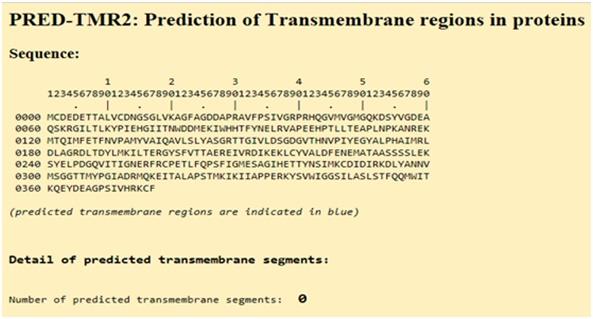
Figure 20:Prediction of trans-membrane regions in Actin
Suggested Protein
Background
Protein Name: Forkhead box protein P3
Gene symbol: FOXP3.
Gene ID: 50943.
UniProtKB/Swiss-Prot: Q9BZS1
Organism: Homo sapiens (Human)
sequence length: 431
Mass (Da): 47,244
Description
Forkhead box protein P3, which is otherwise known as the FOXP3 protein is fundamentally a transcriptional repressor. The FOXP3 protein plays a crucial role in developing and function of the CD4(+)CD25(+) regulatory T cells. The FOXP3 presents itself in the human being in two kinds of isoforms. The first isoform is a full-length form and the other isoform is a smaller form that lacks the exon 2. Only the protein with the full-length form is capable of interacting with the retinoic acid receptor associated orphan receptor (RORα) for inhibiting its ability as an activator of transcription (Du, Huang, Zhou, & Ziegler, 2008).
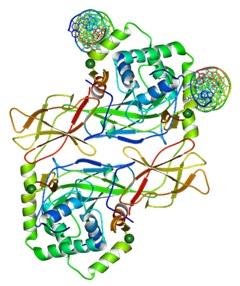
Figure 21:Structure of a domainswapped FOXP3 dimer.
GETTING STUCK WITH SIMILAR ACTIN ASSIGNMENT? ENROL WITH EXPERTSMINDS'S ACTIN ASSIGNMENT HELP SERVICES AND GET DISTRESSED WITH YOUR ASSIGNMENT WORRIES!